| [Chemistry]Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides | ||
|
||
|
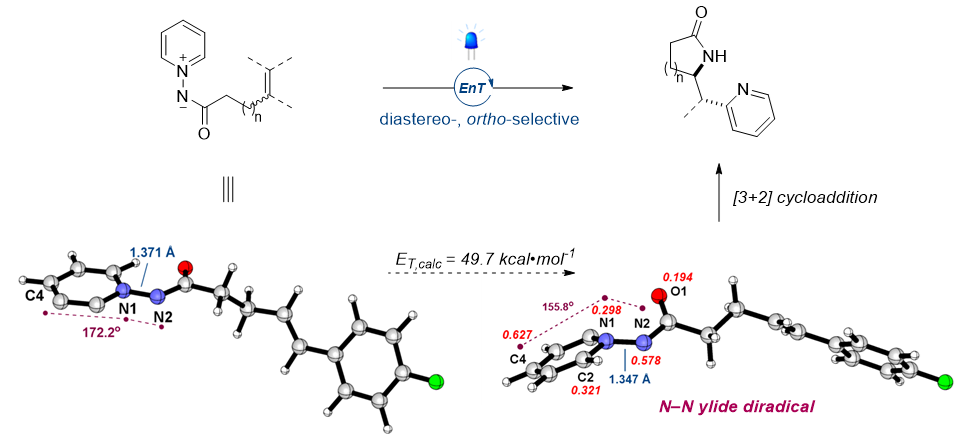
Photocycloaddition offers a potent method for converting alkenes into valuable synthetic materials that are typically difficult to achieve thermally. Current synthetic strategies fall short at combining lactams and pyridines—both vital in pharmaceuticals—within one molecular structure. Our research introduces an efficient, diastereoselective method for pyridyl lactamization via photoinduced [3+2] cycloaddition. This strategy harnesses the unique triplet-state reactivity of N–N pyridinium ylides in the presence of a photosensitizer. The resulting triplet diradical intermediates enable radical [3+2] cycloaddition with a broad range of both activated and unactivated alkenes under mild conditions. This method excels in efficiency, diastereoselectivity, and functional group adaptability, generating ortho-pyridyl γ- and δ-lactam structures with a syn-configuration in a single step. Combined experimental and computational findings confirm that the energy transfer process triggers a triplet-state diradical in N–N pyridinium ylides, paving the way for the cycloaddition.
The formation of triplet state diradicals from the pyridine scaffold was not previously studied. This study tackles this challenge by leveraging an energy transfer mechanism. Computational simulations revealed that the introduction of an amide group to an electron-deficient pyridine could yield an N–N pyridinium salt with a reduced triplet state energy. One significant insight emerged when directly activating the N–N pyridinyl ylide, which led to charge transfer reactivity, transforming it into a diradical. This contrasts with conventional methods as these diradical intermediates can initiate radical formation without the need to break existing groups or add external reagents. Our experiments confirmed the triplet state of pyridinium ylides, unveiling a novel reactivity that concurrently produces compounds containing both lactam and pyridine moieties via a diasteroselective radical [3+2] cycloaddition reaction. This technique, which differs from standard radical shift methods, capitalizes on triplet energy transfer and is compatible with a wide array of functional groups. Notably, it enables the synthesis of lactams without substituents on the amine, obviating the step of substituent removal in N-heteroarenes. Furthermore, this approach is adaptable to various heteroarenes like pyridazine, quinoline, and isoquinoline, and we have effectively integrated ligand-building blocks such as 2,2′-bipyridine and terpyridine. Interestingly, pyridine ylides engage in energy transfer mechanisms with photocatalysts exclusively linked to the carbonyl N–N ylide segment. This suggests the viability of synthesizing diverse ring structures, including a 6-membered lactam with an additional carbon atom, facilitating rapid generation of compound libraries critical in drug development. In sum, this innovative synthesis method, selectively affording the bioactive core structures of lactams and pyridines, provides not only an efficient experimental procedure but also adopts an eco-friendly approach utilizing light. This method holds promising potential for future drug development and various industrial applications.
#triplet state #Energy-transfer #cycloaddition #pyridine #lactam Web address for full article : https://www.nature.com/articles/s41557-023-01258-2 the Name of Journal : Nature Chemistry Laboratory web-address of the author : http://ddnpslab.kaist.ac.kr |
||
|